testing the hardness of water introduction|best water quality test kit : discounter Try this practical with your students to measure the hardness of water samples and investigate the effect of boiling. Includes kit list and safety instructions. WEBSaudi Shemale Alia Alshammary public no hand cum Muslim Khaleji GCC Tgirl Outdoor. 2160p 00:43. 100 %. Nonstop cum load from Venusvero21. Nonstop cum load from Venusvero21. 1080p 01:01. 95 %. redhead trans torso .
{plog:ftitle_list}
webBerichten Sie von Ihren Erfahrungen und lesen Sie die Bewertungen von 46 Kunden. . Dieses Casino heißt halt masonslots und ist genau so schlecht wie die ganzen Online Casinos die es mittlerweilen gibt im Net. . Scheis Betrüger Mason Slots sind die größten bastarden auf dise Erde bitte keine soll bei diesem casino Einzahlung es gebts .

Try this practical with your students to measure the hardness of water samples and investigate the effect of boiling. Includes kit list and safety instructions.Explain the construction and use of a look-up chart. Follow instructions for carrying .
Water hardness is measured in terms of a unit called “parts per million” (ppm) or “milligrams per liter” (mg/L). There are two main methods of measuring water hardness: . Knowing the hardness of your water can help you determine which treatment options are best suited to your needs. In this article, we will explain everything you need to .METHOD SUMMARY. This SOP describes the procedure for measuring hardness by titration with standard EDTA solution to endpoint indicated by a color change. This method is based on . This document describes a student's chemistry investigatory project to test drinking water samples for hardness, iron, fluoride, and chloride. The student outlines procedures to determine hardness using a soap test .
water hardness testing procedure
methods to determine water hardness
In scientific terms, water hardness is generally the amount of dissolved calcium and magnesium in water. But in layman's terms, you may notice water hardness when your .
Water sampling and testing procedures provide information that can be used for the following purposes: to ensure the protection of the water system equipment; to prevent unexpected .Water hardness can be readily determined by titration with the chelating agent EDTA (ethylenediaminetetraacetic acid). This reagent is a weak acid that can lose four protons on .
how to test water hardness at home
Explain the construction and use of a look-up chart. Follow instructions for carrying out a procedure to test hard water. Compare the effectiveness of different water filters. Describe .You will use EDTA complexometric titration to determine the hardness of a sample of water brought from your home. Both the total hardness and the individual calcium and magnesium .titration is testing the hardness of water, for which the method described is an official one (Standard Methods for the Examination of Water and Wastewater, Method 2340C; AOAC Method 920.196). Hardness of water also can be tested by a more rapid test strip method. Such test strips are available from various companies. The strips contain EDTA and A major application of EDTA titration is testing the hardness of water, for which the method described is an official one (Standard Methods for the Examination of Water and Wastewater, Method 2340C; AOAC Method 920.196). Hardness of water also can be tested by a more rapid test strip method. Such test strips are available from various companies.
how to determine water hardness
1.0 Introduction and Applicability Hardness is the presences of dissolved calcium, magnesium and other dissolved polyvalent metal cations in water that can consume soaps used . Water hardness is dominated by the presence of calcium and magnesium Tap water from different parts of the world has different total hardness/ general hardness (GH) and carbonate hardness (KH) levels. GH is a measure of calcium and magnesium ions, while KH is a measure of calcium carbonate anions. pH is a measure of the acidity or basicity of the water. Hence, water quality evaluation becomes necessary and important to ensure the quality of the water. The water quality can be defined as the chemical, physical and biological characteristics of . A major application of EDTA titration is testing the hardness of water, for which the method described is an official one (Standard Methods for the Examination of Water and Wastewater, Method 2340C; AOAC Method 920.196). Hardness of water also can be tested by a more rapid test strip method. Such test strips are available from various companies.
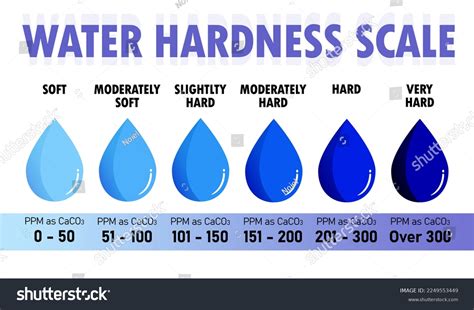
This document defines hardness of water as the property of water to form an insoluble curd with soap instead of lather due to the presence of calcium, magnesium, bicarbonates, sulfates and chlorides. It classifies hardness into temporary and permanent and discusses how hardness is measured in milliequivalents per liter. Hardness levels are . Hardness testing can be applied to various types of materials, some of which are listed below: Metals and alloys. Ceramics. Elastomers. Polymers and plastics. Films. Rocks and minerals. Importance of Hardness Testing. Hardness testing is important for a number of different reasons. The hardness of a material can be a critical parameter in its .concentrations. Total water hardness is usually expressed as the milligrams of CaCO3 equivalent to the total amount of calcium and magnesium present in one liter of water (mg/liter, i.e., ppm). Water hardness may range from zero to hundreds of ppm, depending on the source. The classification of degree of water hardness
Mohs Hardness Test: This qualitative test ranks minerals based on their scratch resistance against standard materials with known hardness values. It’s commonly used in geology and mineralogy for mineral identification. These hardness testing methods serve various purposes, from quality control in manufacturing processes to material selection .Introduction: Hardness in water is caused by dissolved minerals, primarily divalent cations, including calcium(Ca2+), iron (Fe2+), strontium (Sr2+), zinc (Zn2+) and manganese (Mn2+). . The traditional test for hardness involves pH adjustment to 10.1 with an ammonium buffer, addition of Eriochrome Black T indicator [1-(1- hydroxy-2-naphthylazo .
hardness of water testing method
First, you have to measure the water hardness with a water hardness test. You can get it online for less than . If you measure that your water hardness is below 60 ppm or 3.50 GPG, you don’t have to worry about hard water. By classification, you have soft water.

specific gravity test using density bottle method
Introduction What Is Rockwell Hardness Test? The Rockwell Hardness Test is one of several tests used to determine whether a material is solid and durable enough to be employed as a component of an object. The Knoop, Brinell, and Vickers procedures are additional examinations in the sequence. Process The Rockwell Hardness test and its . As water hardness is usually reported in terms of mg/L of calcium carbonate (even if water contains both calcium and magnesium), we will use for calculations slightly strange reaction equation: CaCO 3 + EDTA 4-→ CaEDTA 2-+ CO 3 2-That allows direct calculation of calcium carbonate mass for known amount of titrant used.
Water testing is a broad description for various procedures used to analyze water quality. Millions of water quality tests are carried out daily to fulfill regulatory requirements and to maintain safety. . See Bacteriological water analysis and specific tests such as turbidity and hard water. "finished" water quality – water treated at a .
specimen bottle for spinal fluid test
Testing the hardness of water The concentration of dissolved calcium ions (otherwise known as the ‘hardness’ of the water) can be determined by taking one kilogram of water (approximately one litre) and finding the mass of dissolved calcium ions in milligrams. A 1 kg sample of water containing 0.100 g (100 mg) of calcium ions has a To avoid the adverse effects of hard water, the calcium and magnesium mineral content in the water should be below 60 mg/L. The USGS standards for water hardness levels are as follows: Soft water: 0 to 60 mg/L; . Hardness: Water hardness refers to the presence of calcium and magnesium salts in water. Temporary hardness is caused by carbonate and bicarbonate ions, while permanent hardness is due to chloride and sulfate ions. Hard water can have certain benefits for drinking purposes, but excessive levels of certain ions can be a nuisance because of scale .
The unknown sample had a CaCO 3 average ppm of 98. which is 98 mg/L, therefore meeting the Toronto Drinking Water Standards. (City Council, Chemicals in Drinking Water) The water softening process begins as hard water enters the water softener machine with resin beads that attract and hold onto hard water minerals, removing them from the water.
One of the factors that establishes the quality of a water supply is its degree of hardness. The hardness of water is defined in terms of its content of calcium and magnesium ions. Since an analysis does not distinguish between Ca2+ and Mg2+, and since most hardness is caused by carbonate depositsINTRODUCTION Water hardness is the traditional measure of the capacity of water to precipitate soap. Hard water requiring a considerable amount of soap to produce leather. Scaling of hot water pipes, boilers and other house hold appliances is due to hard water. Hardness of water is no specific constituent but is a variable and complex mixture .Testing water for hardness Introduction Water that is described as "hard" has high amounts of dissolved minerals, specifically calcium and magnesium. Hard water is not a health risk. The calcium in hard water may actually be beneficially for growing bones. However, hard water can be a nuisance because of poor soap performance. Temporary Hard Water. Temporary hard water is hard water that consists primarily of calcium (Ca 2 +) and bicarbonate (HCO 3-) ions.Heating causes the bicarbonate ion in temporary hard water to decompose into carbonate ion (CO 3 2-), carbon dioxide (CO 2), and water (H 2 O). The resultant carbonate ion (CO 3 2-) can then react with other ions in the .
Introduction Total Hardness in water is determined using the preprogrammed method, T7A Total Hard. To determine total hardness, ammonia buffer is added to a sample . Leave the fill hole open during testing. Rinse thoroughly with RGW before and between titrations. At the end of the day, thoroughly rinse the electrode with RGW and store in .Introduction. Complexometric titrations are used to indicate the end point of a titration based on the formation of a colored complex. . Based off this data, the experiment results for water hardness should be accurate (considering the lab was done in Mesa) and it can be concluded that Mesa’s water is considered “hard” since it exceeds .
Water Hardness Testing 19 by Complexometric Determination of Calcium S. Suzanne Nielsen Contents 19.1 Introduction 157 19.1.1 Background 157 19.1.2 Reading Assignment 158 19.1.3 Objectiev 158 19.2 EDTA Titrimetric Method for Testing Hardness of Water 158 19.2.1 Principle of Method 158 19.2.2 Chemicals 158What is hard water? Hard water is caused by the presence of naturally occurring calcium and magnesium salts in water. Water hardness is usually noticed because of difficulty in lathering soap and the formation of a scum when washing. Calcium and magnesium ions (Ca2+ and Mg2+) form insoluble salts with soaps causing precipitation of this soap scum.
spray bottle hair porosity test
spray bottle mask test
Espaço Itaú de Cinema - Frei Caneca. R. Frei Caneca, 569 - Bela Vista. Ver no mapa. Sessões. Detalhes. 22/02 Hoje. 23/02 sex. 24/02 sáb.
testing the hardness of water introduction|best water quality test kit